Although there are hundreds of different tooth whitening methods and products available on the market, the most effective bleaching agents all have something in common. They either contain Carbamide Peroxide (which breaks down to Hydrogen Peroxide) or they contain Hydrogen Peroxide.
How do peroxide teeth whiteners work?
- Peroxide is an oxidizing agent.
- The peroxide breaks down in the mouth and releases highly reactive molecules called free radicals.
- These molecules penetrate the porosities in the rod-like crystal structure of enamel and breaks down the stain deposits in the enamel and even dentin.
- As the resulting byproducts are colorless – the teeth ends up looking whiter.
- If you are using Carbamide Peroxide, it will break down to hydrogen peroxide, which then releases the free radicals. The advantage of using Carbamide Peroxide is that it is more stable and helps to insure the product quality at the consumer’s end. It is also beneficial to use the Carbamide Peroxide that takes more time to transform and release the free radicals as it will be effective for a longer, and at a more sustained rate. An example is Opalescence PF. Hydrogen peroxide is great for quick, in-office bleaching as it is very effective, but only for a short period of time e.g. Zoom.
Other ingredients in whitening gels
- Gelling agents: They add emulsifying agents to get a consistency that is runny enough to spread and cover the teeth properly but also thick enough to stay in the bleaching tray.
- Stabilizers: Peroxides are relatively reactive compounds and tend to degrade over time, making it less effective. As the shelf life should preferably be at least a year, most whiteners contain stabilizers. However, it is still a good idea to store your bleaching gel in the refrigerator.
- Buffering agents: The pH of the whitening gel should be close to neutral (pH 7.0) as the chance of tooth sensitivity or even damage to the teeth if higher if the whitener is too acidic. The buffering agents help to keep the gel’s pH within an acceptable range.
- Flavoring: Many whitening gels include flavoring agents.
- Desensitizing Agents: As tooth sensitivity is a common complaint when people bleach their teeth, some manufacturers include a desensitizing agent in their product. In most case it is potassium nitrate or fluoride.
Safety of Carbamide Peroxide
Systemic Toxicity
If someone swallows a large quantity of Carbamide Peroxide or Hydrogen Peroxide it will certainly be harmful. The product should therefore be store out of the reach of children. If you use the product according to the instructions and spit out the bleaching agent after use, rather than swallowing it, it is perfectly safe. Over 25 years of using this bleaching technique, no adverse systemic effects have been reported.
Pregnant women and children
 Due to a lack of research evidence, the American dental Association recommends against whitening treatments during pregnancy, while nursing and for young children. I would suggest that it is better not to bleach teeth for children under 10 using tray whitening. Even then, the dentist and parent’s close supervision is necessary.
Due to a lack of research evidence, the American dental Association recommends against whitening treatments during pregnancy, while nursing and for young children. I would suggest that it is better not to bleach teeth for children under 10 using tray whitening. Even then, the dentist and parent’s close supervision is necessary.
Damage to Teeth due to Carbamide Peroxide
There has been some research showing changes to the tooth enamel, including increased surface roughness, increase porosity and loss of microhardness. The good news is that Carbamide Peroxide has been in use for 25 years by the general population and has not demonstrated to be a major problem.
I would suggest using a 10% concentration Carbamide Peroxide for at-home tray-whitening, rather than some of the much higher concentration products on the market, as it will minimize the risk of damage to hard and soft tissue and tooth sensitivity. Whiteners with fluoride may also help to minimize the risk of enamel damage.
There have been cases of tooth damage due to over-the–counter products which not only contained a high peroxide concentration but were very acidic.
Damage to Soft tissue
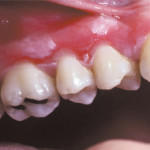
Hydrogen peroxide burn
Yes, Carbamide Peroxide and Hydrogen Peroxide can cause damage to soft tissue. The free radicals released by the peroxide compounds are known to interact with proteins and lipids and cause cellular damage. The bleaching gel can be corrosive to mucous membranes and sort tissue irritation is unfortunately a common side effect of using Carbamide Peroxide or Hydrogen Peroxide. If you use 10% Carbamide Peroxide (rather than a stronger product) and whipe any access gel that pushes out when placing the trays in your mouth, you shouldn’t get any long-term ill effects.




